Performing Test & Interpretation
The RapCov™ COVID-19 Rapid test is a lateral flow immunochromatographic assay. The 2019-nCoV recombinant nucleocapsid protein is used to detect antibodies to 2019-nCoV in fingerstick whole blood from patients who are suspected of COVID-19 virus infection.
To use the RapCov™ COVID-19 Rapid test, the device cassette, specimen, and buffer solution are allowed to equilibrate to room temperature.
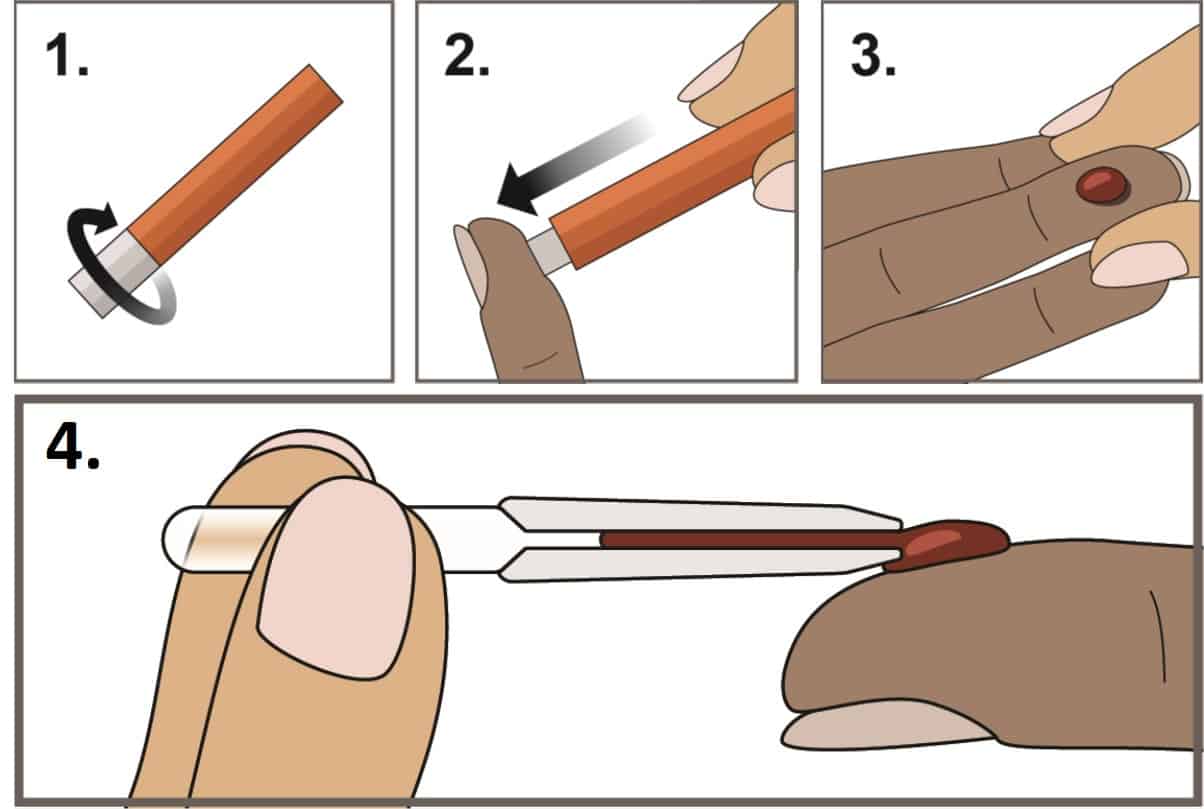
Step 1: Fingerstick whole blood collection: Blood sample is obtained by fingerstick using TRUEplus Safety Lancet and collected using MicroSafe® pipette Specimen (10 μL). The health care provider washes hands thoroughly and put on gloves. Select the site: The patient should be sitting or lying down. The health care provider holds patient’s hand in a downward position, allowing gravity to help increase blood supply to the hand and selects middle or ring finger for fingerstick. The health care provider twists off the tab of to break the seal and discard the cap and performs the puncture by positioning the safety lancet firmly against the puncture site. The lancet is activated by pressing firmly against the puncture site. A blood drop forms at the puncture site. The used safety lancet is discarded into a sharps container or according to the health facility’s established procedures.
Product page for TRUEplus Safety Lancet →
The whole blood specimen is collected using MicroSafe® pipette. The pipette is held horizontally to touch the tip to the blood sample. Capillary action automatically draws the sample blood to the air vent and it will stop.
Video Demonstration of MicroSafe® pipette use →
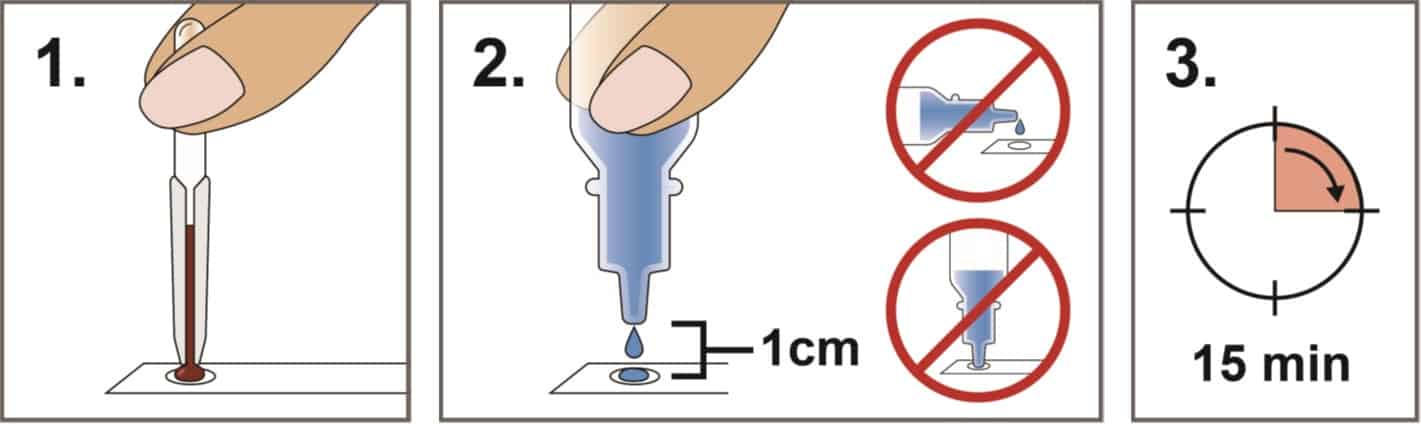
Step 2: Transfer Blood specimen to Test Cassette well: Place the test cassette on a horizontal hard surface. To expel blood, align the tip of the pipette with the test cassette well and squeeze the bulb.
Step 3: Apply Buffer to Test Cassette well: After the sample well is free of blood, two drops of Buffer are then added to the sample well.
Step 4: Test Readout: Wait for fifteen minutes and read the test results. Results are not to be read after twenty minutes.
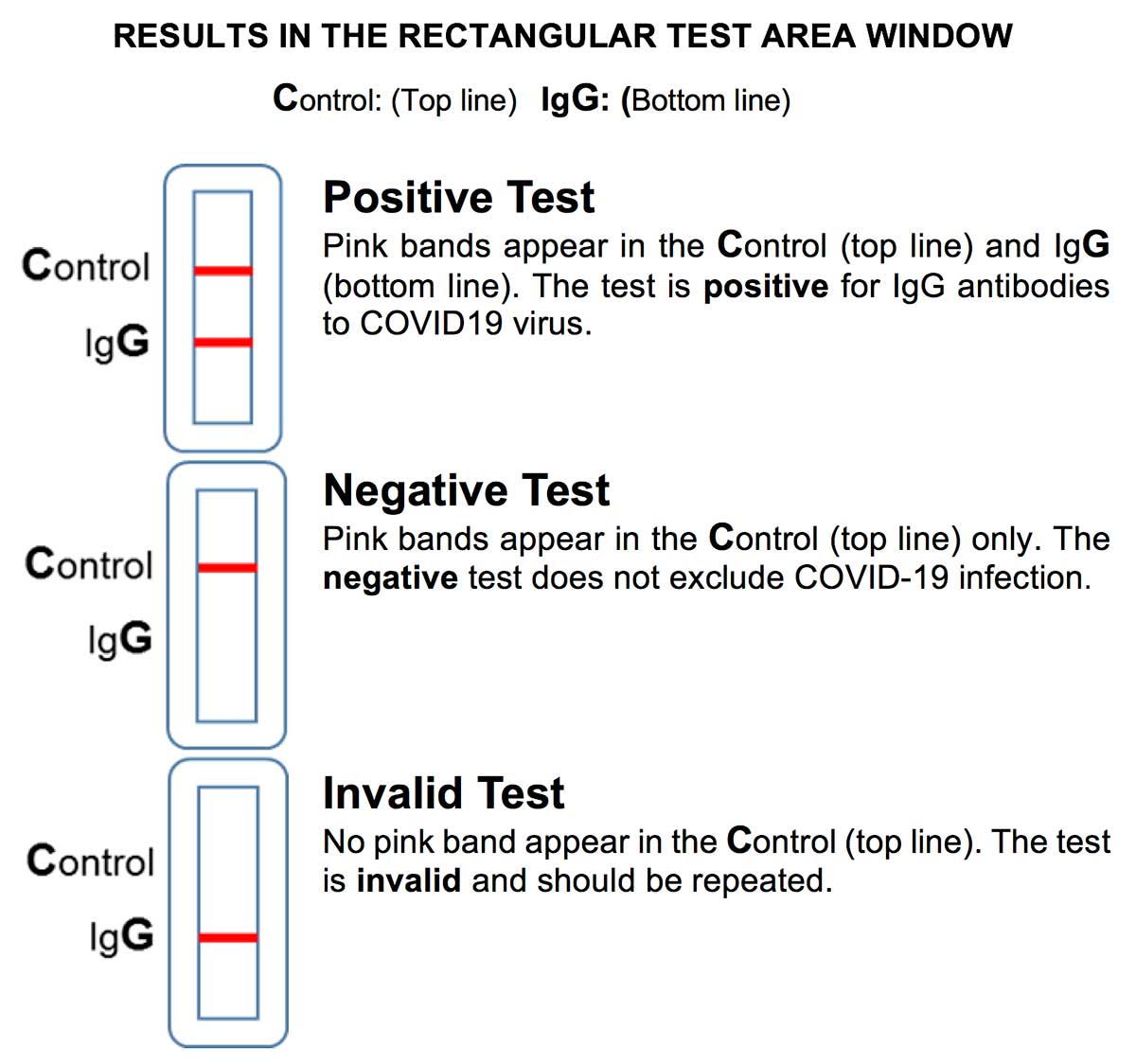
Examination and Interpretation of Patient Specimen Results
The RapCov™ COVID-19 Rapid Test must be performed as described in the Instructions For Use (IFU) document. Assessment of clinical specimen test results should be performed after the internal control has been examined and determined to be valid and acceptable. If the internal control is not valid, the patient results cannot be interpreted.
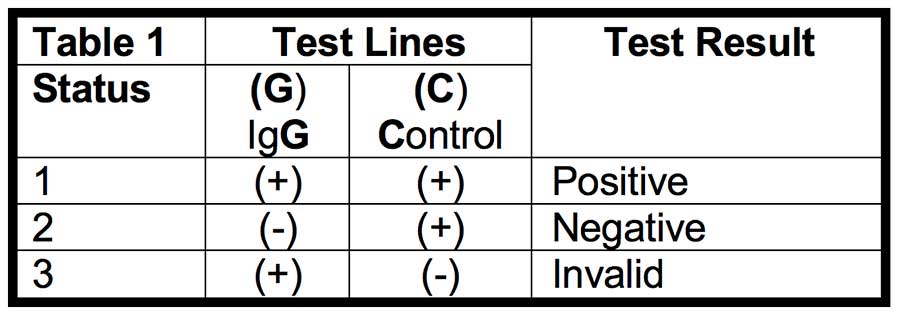
To use the RapCov™ COVID-19 Rapid Test, the device cassette, specimen, and buffer solution are allowed to equilibrate to room temperature. Fingerstick whole blood specimen (10 μL) is transferred to the center of the sample well. After the sample well is free of liquid, two drops of Buffer are then added to the sample well. Wait for fifteen minutes and read the test results. Results are not to be read after twenty minutes. Positive Result occurs when a pink colored band appears at both the IgG Test Line (G) and Control Line (C) and indicates that antibodies against SARS-CoV-2 are present (IgG); and. A Negative Result occurs when a colored band appears at Control Line (C) only and indicates that antibodies against SARS-CoV-2 were not detected. An Invalid Result occurs when no colored band occurs at Control Line (C) and the test should be repeated.
Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
Negative results do not preclude 2019-nCoV infection and should not be used as the sole basis for patient management decisions. Negative results must be combined with clinical observations, patient history, and epidemiological information. Risks to a patient of a false negative result include: delayed or lack of supportive treatment, lack of monitoring of infected individuals and their household or other close contacts for symptoms resulting in increased risk of spread of COVID-19 within the community, or other unintended adverse events.
Positive results are presumptive and must be confirmed by virus isolation or viral nucleic acid detection by RT-PCR for confirmation of COVID-19 virus infection. Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.