COVID-19 Virus Disease
There has been an outbreak of respiratory disease caused by a novel coronavirus that was first detected in China and which then spread to several locations internationally, including in the United States. The virus named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), also known as “the COVID-19 virus”, causes the Coronavirus Disease (COVID-19). The WHO declared COVID-19 disease to be a pandemic in early March. Reported illnesses have ranged from very mild (including some with no reported symptoms) in the majority of the cases (80%) to severe in 20% cases, including illness resulting in death (in 1-4% cases).
There is no single ‘Gold Standard’ for the diagnosis of COVID-19 infection. The different diagnostic methodologies provide different information regarding COVID-19 infection and a complete clinical picture is made possible by using information provided by all testing methodologies.
CDC FAQs regarding COVID-19 disease →
Molecular Testing for COVID-19
COVID-19 disease diagnosis can be made in the viremia phase of the disease (during incubation period and first week of the disease since onset of symptoms) by detecting the virus nucleic acid using the RT-PCR. The RT-PCR test is a molecular-based test that supports diagnosis of an acute infection due to COVID-19 virus to inform patient care decisions and infection control actions to prevent spread of the disease. Generally, the RT-PCR test is performed using a nasopharyngeal swab.
Serological Testing for COVID-19
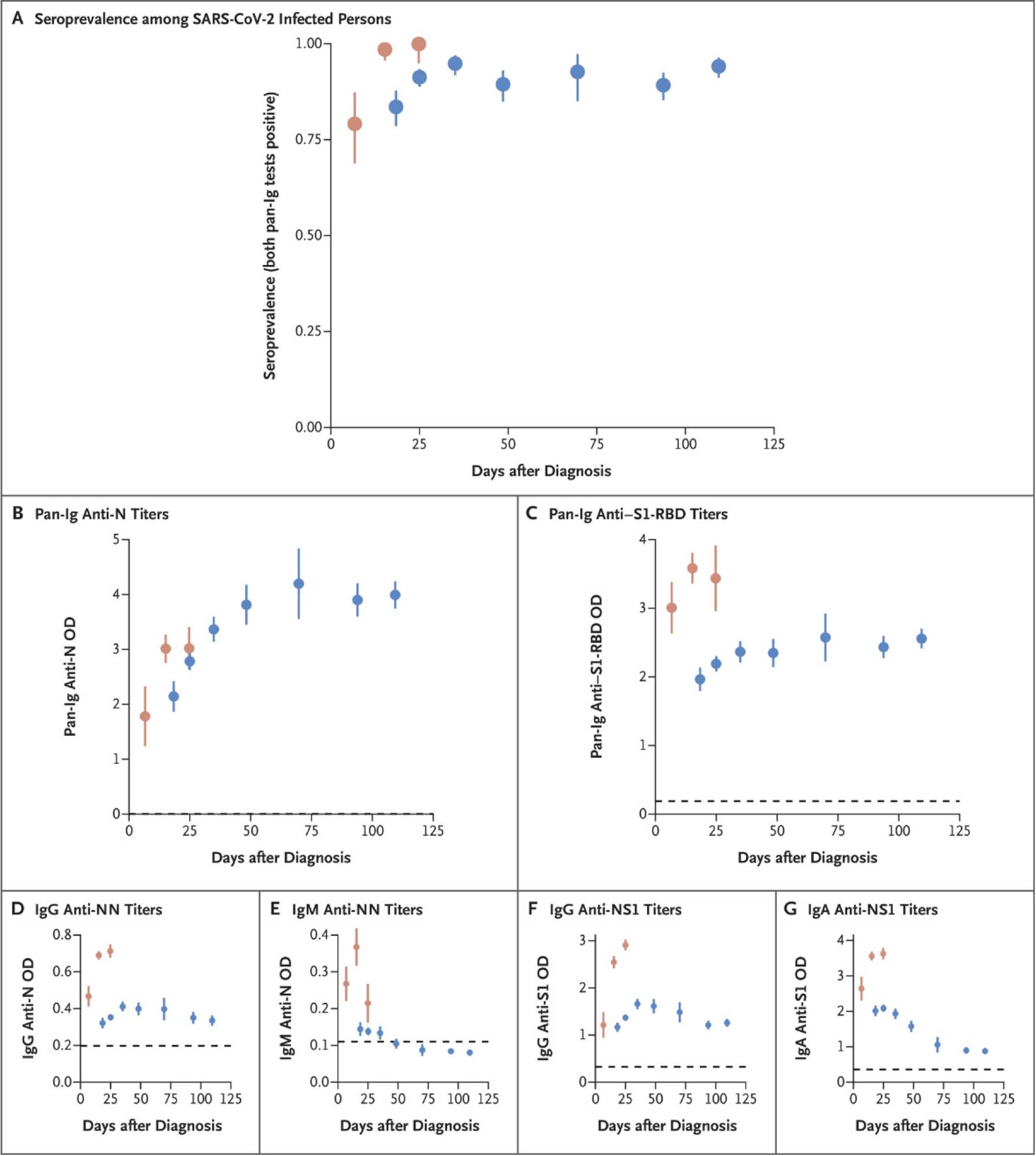
Nearly all immune-competent individuals will develop an immune response following SARS-CoV-2 infection. Like infections with other pathogens, SARS-CoV-2 infection elicits development of IgM and IgG antibodies, which are the most useful for assessing antibody response because little is known about IgA response in the blood.
Antibodies in some persons can be detected within the first week of illness onset. In SARS-CoV-2 infections, IgM and IgG antibodies can arise nearly simultaneously in serum within 2 to 3 weeks after illness onset. Thus, detection of IgM without IgG is uncommon. How long IgM and IgG antibodies remain detectable following infection is not entirely known. It is also important to note that some persons do not develop detectable IgG or IgM antibodies following infection. Thus, the absence of detectable IgM or IgG antibodies does not necessarily rule out that they could have previously been infected.
A study on 30,576 persons evaluating the humoral immune response in Iceland indicated that antiviral antibodies against SARS-CoV-2 did not decline within 4 months after diagnosis. In specific, IgG antibody levels increased during the first 6 weeks after diagnosis; while IgM antibody levels increased rapidly soon after diagnosis and then fell rapidly and were generally not detected after 2 months.¹
RapCov™ COVID-19 Rapid Test is a lateral flow immunochromatographic assay for the presumptive qualitative detection of IgG antibodies to the COVID-19 virus in human whole blood fingerstick samples.
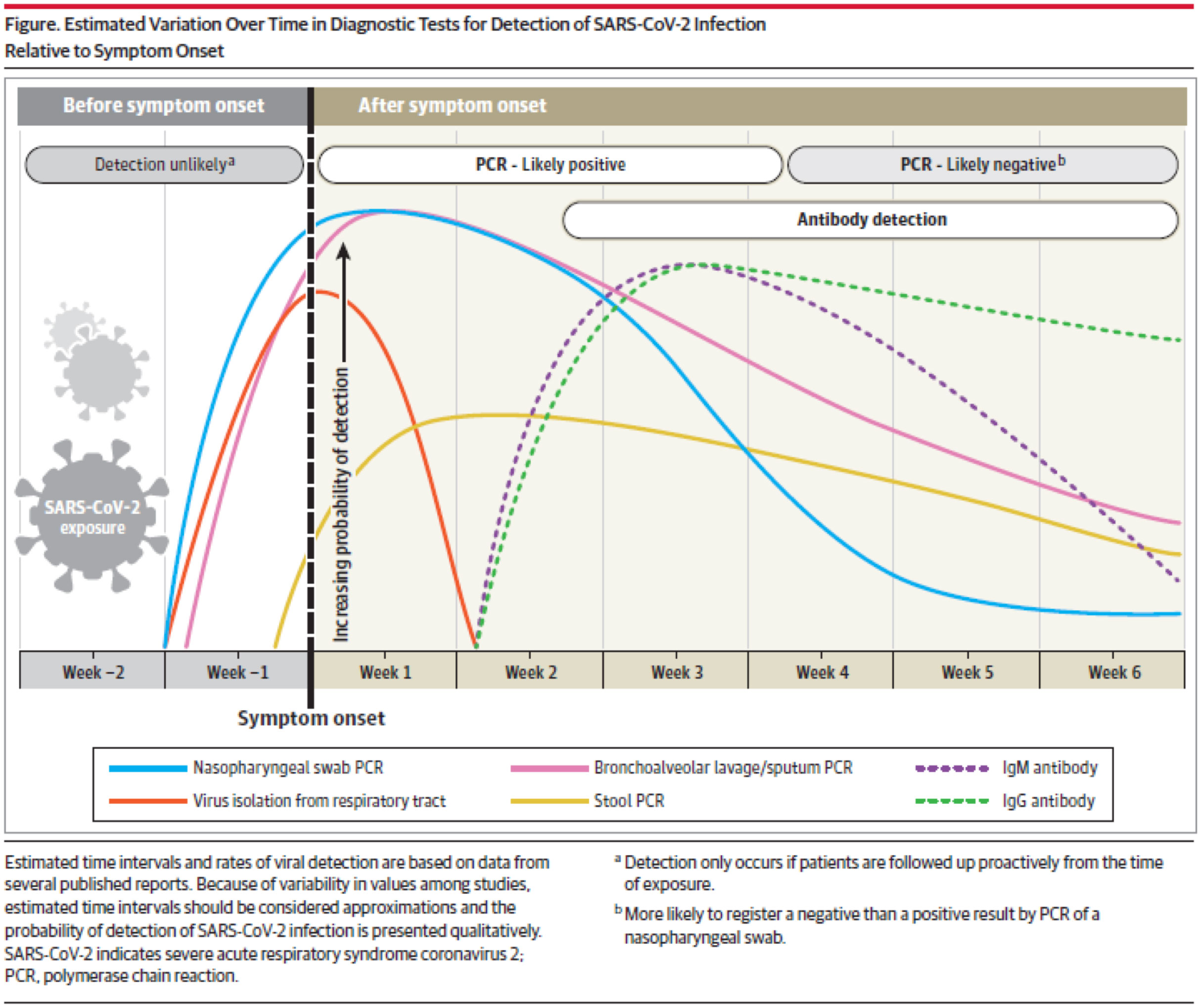
Detection of SARS-CoV-2 Infection Relative to Symptom Onset ² (Green Line = IgG)
High titers of IgG antibodies have been shown to positively correlate with neutralizing antibodies.³
Specific Public Health and Medical Uses of Rapcov™ COVID-19 Rapid Test
There exists a great need for laboratory assays that measure antibody responses. While serological assays are not well suited to detect acute infections, they support a number of highly relevant public health and medical applications.
- From a community perspective to limit the spread of infection, the Rapcov™ test may help in the following practical ways:
- Presence of IgG antibodies on Rapcov™ test may suggest a non-recent (past) infection as neutralizing antibodies against the COVID-19 virus may be present in the blood. Presence of COVID-19 virus neutralizing IgG antibodies will suggest that the individual is of little concern from a public health perspective and is perhaps unlikely to get infected with COVID-19 virus. Presence of IgG antibodies on Rapcov™ test will also permit to determine who is immune and who is not. This would be very useful for deploying immune healthcare workers in a strategic manner as to limit the risk of exposure and spread of the virus inadvertently.
- In cases where nucleic acid amplification assays (RT-PCR) are negative and there is a strong epidemiological link to COVID-19 infection, serology tests (in the acute and convalescent phase) may support diagnosis of COVID-19 disease.
- Serosurveys are needed to determine the precise rate of infection in an affected area, which is an essential variable to accurately determine the infection fatality rate. Serological surveys can aid investigation of an ongoing outbreak and retrospective assessment of the attack rate or extent of an outbreak.
- From a medical decision-making perspective the information regarding presence of IgG antibodies in the blood may help save lives in the following ways:
- It may help medical decision making in the treatment of acutely ill hospitalized patients who have CT scan and other clinical findings consistent with COVID-19 disease, but a negative RT-PCR result. In these patients Rapcov™ test may provide information regarding presence of IgG antibodies to support a diagnosis of COVID-19 infection and facilitate appropriate medical treatments. There is no single ‘Gold Standard’ for the diagnosis of COVID-19 infection. The different diagnostic methodologies provide different information regarding COVID-19 infection and a complete clinical picture is made possible by using information provided by all testing methodologies.
- Currently there are no specific anti-viral treatments for COVID-19 disease. The RapCov™ test administered in the communities can identify individuals who mounted strong IgG antibody responses and who could serve as donors for the generation of convalescent serum therapeutics. This serum can be administered for prophylaxis and treatment of COVID-19 infection to save lives.
¹ Alter Galit, Seder Robert. (2020) The Power of Antibody-Based Surveillance. N Engl J Med DOI: 10.1056/NEJMe2028079.
² JAMA. 2020;323(22):2249-2251. doi:10.1001/jama.2020.8259
³ To KK-W, Tsang OT-Y, Leung W-S, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565-574. doi:10.1016/S1473-3099(20)30196-1
