RapCov Clinical Studies
STUDY #1: Clinical Performance Evaluation
-
- Methodology: The evaluation was performed in US using fingerstick whole blood specimens (n=30) from patients who had COVID-19 disease confirmed by an EUA-authorized RR-PCR test. Fingerstick whole blood samples presumed to be negative (n=104) were obtained from healthy subjects. Fingerstick whole blood samples were tested using RapCov™ Rapid COVID-19 Test per the manufacturer’s Instruction for Use (IFU). Positive RapCov™ Rapid COVID-19 test was defined as presence of IgG COVID19 antibodies.
- Results: Positive Percent Agreement (PPA) was 90% with RT-PCR positive SARS-CoV-2 infection status and Negative Percent Agreement (NPA) was 95.2%.
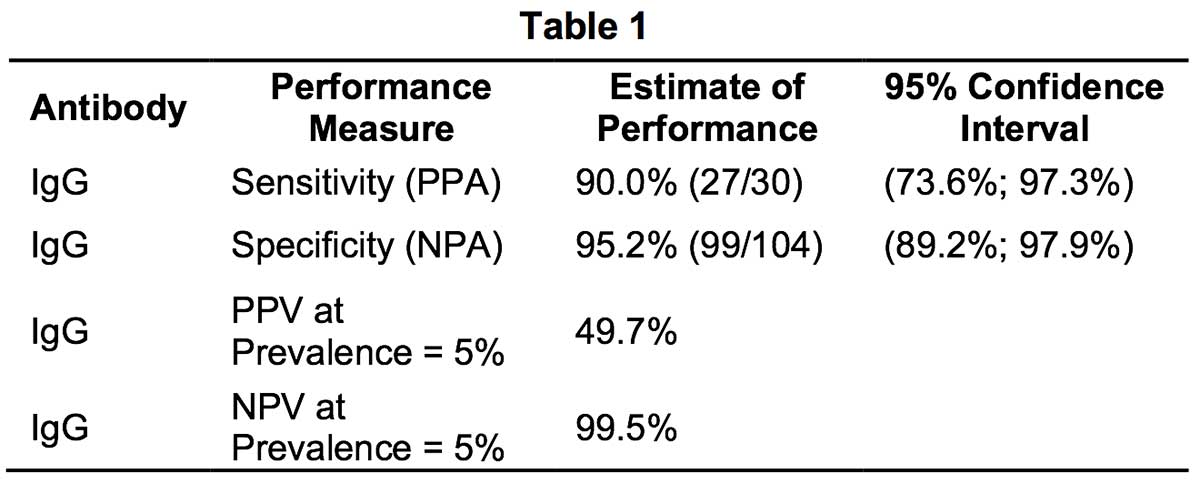
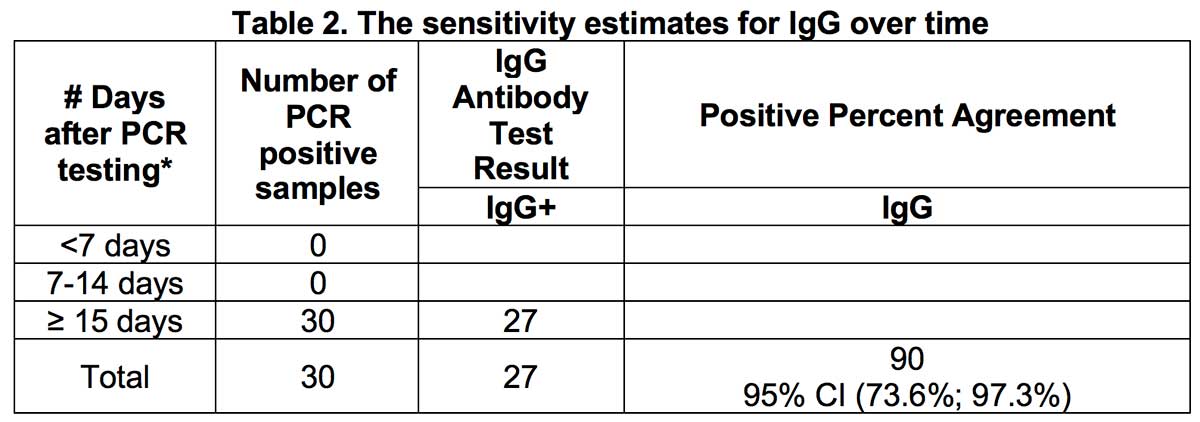
When estimating the sensitivity of IgG over time from symptom onset for all positive samples, the proportion of IgG positive patients was 90% approximately 15 days after symptom onset.
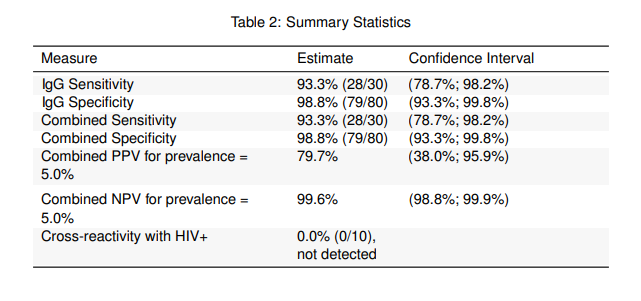
STUDY #2: NCI/NIH Independent Evaluation Study
STUDY #3: Cross-Reactivity Evaluation
-
-
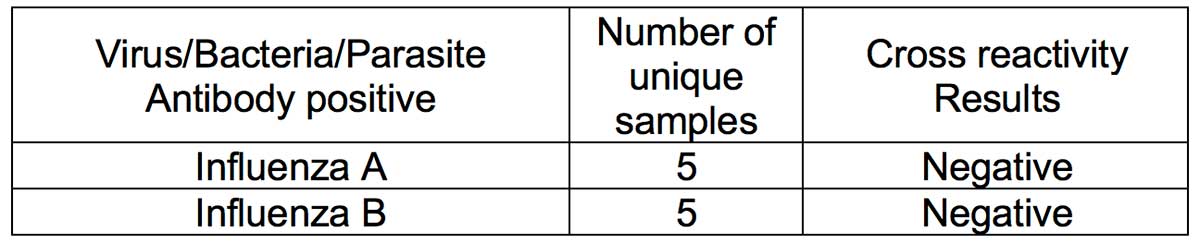
- Methodology: A cross-reactivity evaluation was performed using human serum specimen from patients who had human coronavirus OC43 infection (n=14). Serum samples of patients who had high priority organisms were obtained and cross-reactivity experiments performed. Serum samples containing antibodies to the following viruses were obtained: (i) Influenza A; (ii) Influenza B; (iii) anti-HBV; (iv) anti-HCV; (v) Antinuclear antibodies (ANA); (vi) Haemophilus Influenzae; (vii) Rhinovirus; (viii) anti-respiratory syncytial virus; and (ix) anti-HIV.
-
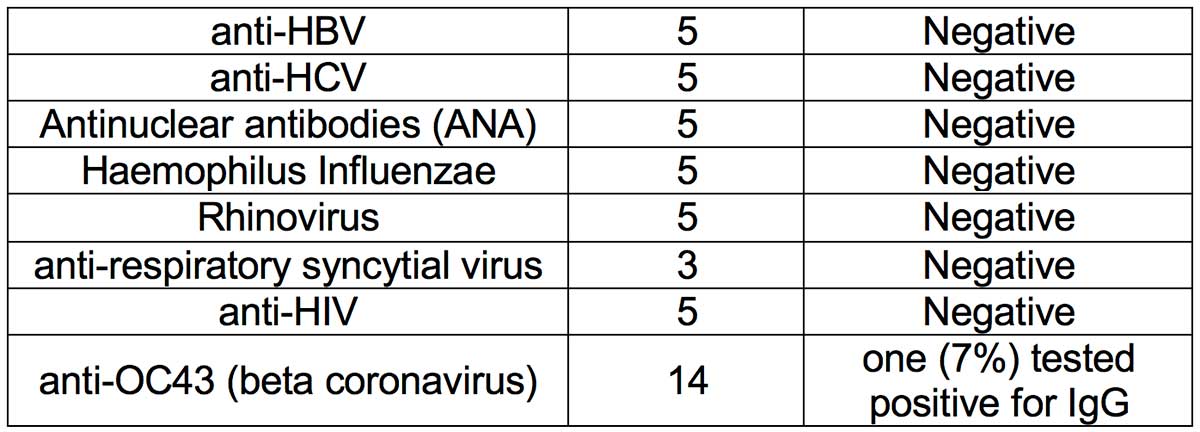
-
- Results: Thirteen (93%) OC43 samples tested negative for IgG, thus showing minimal cross-reactivity with coronavirus OC43 infection. Serum specimen from all tested high priority organisms (Influenza A, influenze B, anti-HBV, anti-HCV, ANA and Haemophilus Influenzae, Rhinovirus, anti-respiratory syncytial virus, and anti-HIV) were negative. Thus, no crossreactivity was observed with the tested high priority organism serum specimens.
